***
WARNING:
INTRAVASCULAR HEMOLYSIS (IVH) – This warning does not apply to Rho(D)- negative patients treated for the suppression of Rh isoimmunization.
Intravascular hemolysis leading to death has been reported in patients treated for ITP (immune thrombocytopenia) with WinRho® SDF. IVH can lead to clinically compromising anemia and multi-system organ failure including acute respiratory distress syndrome (ARDS). Serious complications including severe anemia, acute renal insufficiency, renal failure, and disseminated intravascular coagulation (DIC) have also been reported. Closely monitor patients treated with WinRho® SDF for ITP in a healthcare setting for at least eight hours after administration. Perform a dipstick urinalysis to monitor for hematuria and hemoglobinuria at baseline and 2 hours, 4 hours, and prior to the end of the monitoring period. Alert patients and monitor the signs and symptoms of IVH including back pain, shaking chills, fever, and discolored urine or hematuria. Absence of these signs and/or symptoms of IVH within eight hours does not indicate IVH cannot occur subsequently. If signs and/or symptoms of IVH are present or suspected after WinRho® SDF administration, post-treatment laboratory tests should be performed including plasma hemoglobin, haptoglobin, LDH, and plasma bilirubin (direct and indirect). If ITP patients are to be transfused after receiving WinRho® SDF, use Rho(D)-negative red blood cells so as not to exacerbate ongoing hemolysis.
Indication and Usage:
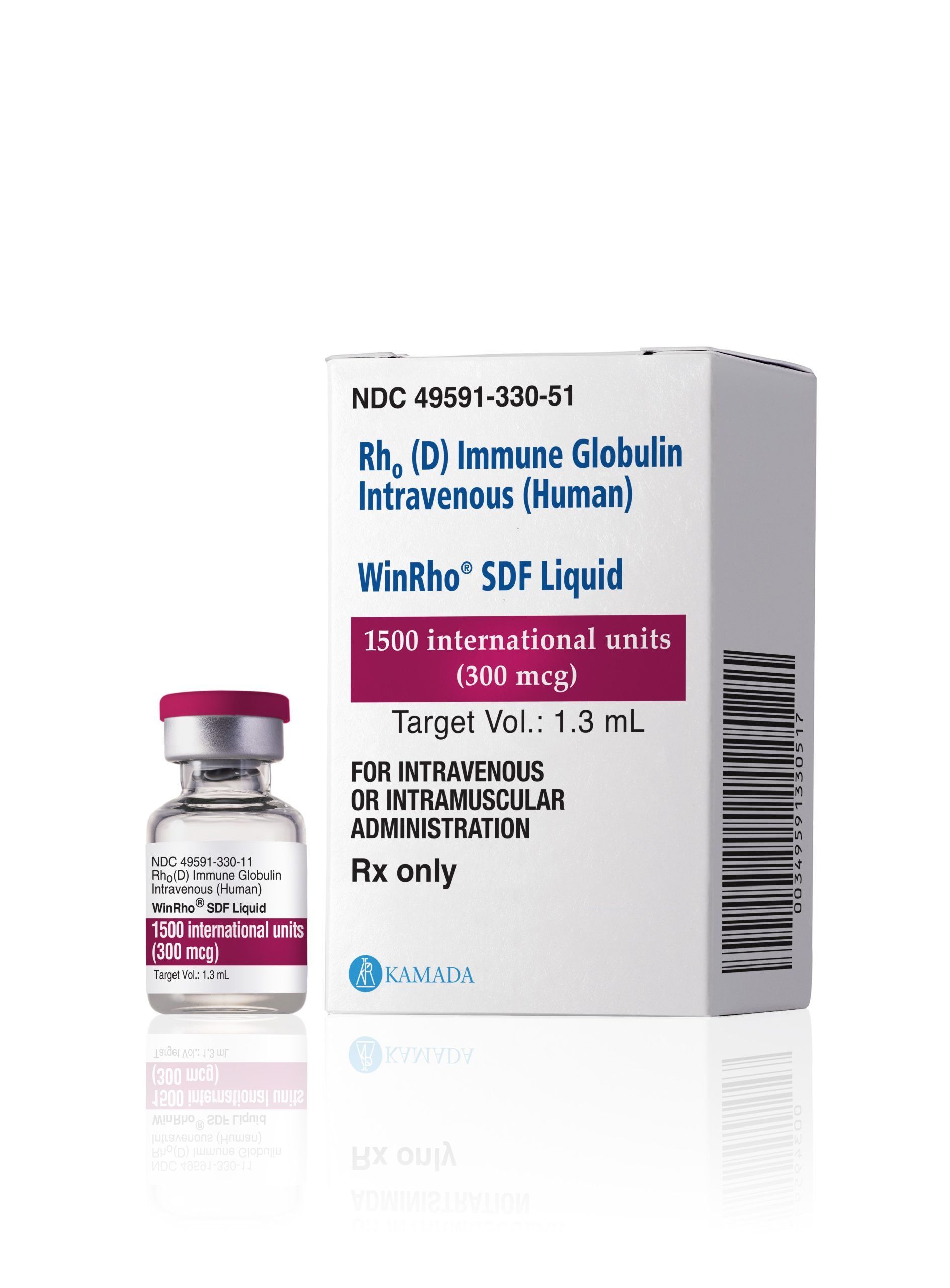
WinRho® SDF is a Rho(D) Immune Globulin Intravenous (Human) product indicated for the treatment of immune thrombocytopenia (ITP) in Rho(D)-positive patients and for the suppression of Rh isoimmunization in non-sensitized Rho(D)-negative patients
The safety and efficacy of WinRho® SDF have not been evaluated in clinical trials for patients with non-ITP causes of thrombocytopenia or in previously splenectomized patients or in patients who are Rho(D)-negative.
Pregnancy and Other Obstetric Conditions
WinRho® SDF is indicated for the suppression of Rh isoimmunization in non-sensitized, Rho(D)-negative (D-negative) women with a Rh-incompatible pregnancy, including:
A Rh-incompatible pregnancy is assumed if the fetus/baby is either Rho(D)-positive or Rho(D)-unknown or if the father is either Rho(D)-positive or Rho(D)-unknown.
Incompatible Transfusions
WinRho® SDF is indicated for the suppression of Rh isoimmunization in Rho(D)-negative individuals transfused with Rho(D)-positive red blood cells (RBCs) or blood components containing Rho(D)-positive RBCs.
WinRho® SDF is not indicated for use as immunoglobulin replacement therapy for immune globulin deficiency syndromes.
Important Safety Information:
WinRho® SDF is contraindicated in:
Patients who have had known anaphylactic or severe systemic reaction to the administration of
human immune globulin products, IgA deficient patients with antibodies to IgA and a history of hypersensitivity reaction to WinRho® SDF or any of its components, Patients with autoimmune hemolytic anemia, with pre-existing hemolysis or at high risk for hemolysis, Infants for the suppression of Rho(D) isoimmunization.
The liquid formulation of WinRho® SDF contains maltose. Maltose is a disaccharide sugar derived from corn. Patients with corn allergy should avoid using WinRho® SDF due to risk of hypersensitivity. In addition, Maltose in IGIV products has been shown to give falsely high blood glucose levels in certain types of blood glucose testing systems. Due to the potential for falsely elevated glucose readings, only use testing systems that are glucose-specific to test or monitor blood glucose levels in patients receiving WinRho® SDF Liquid.
WinRho® SDF is made from human plasma. It may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Severe hypersensitivity reactions may occur. If symptoms of allergic or early signs of hypersensitivity reactions (including generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis) occur, discontinue WinRho® SDF infusion immediately and institute appropriate treatment.
Acute renal insufficiency /failure, osmotic nephropathy, acute tubular necrosis, proximal tubular nephropathy, and death may occur upon use of Immune Globulin Intravenous (IGIV) products, including WinRho® SDF. Ensure that patients are not volume depleted before administering WinRho® SDF. For patients at risk of renal insufficiency or failure, including those with any degree of pre‑existing renal insufficiency, diabetes mellitus, advanced age (above 65 years of age), volume depletion, sepsis, paraproteinemia, or receiving known nephrotoxic drugs, administer WinRho® SDF at the minimum infusion rate practicable and assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of WinRho® SDF and at appropriate intervals thereafter.
Although the mechanism of action of WinRho® SDF in the treatment of ITP is not completely understood it is postulated that anti-D binds to the Rho(D) RBC resulting in formation of antibody-coated RBC complexes. Immune-mediated clearance of the antibody-coated RBC complexes would spare the antibody-coated platelets because of the preferential destruction of antibody-coated RBC complexes by the macrophages located in the reticuloendothelial system. The side effect of this action is a decrease in hemoglobin levels (extravascular hemolysis).
Thromboembolic events may occur following treatment with WinRho® SDF and other IGIV products. Patients at risk include those with a history of atherosclerosis, multiple cardiovascular risk factors, advanced age, impaired cardiac output, coagulation disorders, prolonged periods of immobilization, history of arterial or venous thrombosis, estrogen use, indwelling central vascular catheters, and/or known/suspected hyperviscosity. Thrombosis may occur in the absence of known risk factors.
Non-cardiogenic pulmonary edema [Transfusion-related Acute Lung Injury (TRALI)] may occur in patients following IGIV treatment, including WinRho® SDF.
WinRho® SDF should not be administrated to Rho(D)-negative individuals who are Rh immunized as evidenced by an indirect antiglobulin (Coombs’) test revealing the presence of anti-Rho(D) (anti-D) antibody.
The most common adverse reactions of WinRho® SDF include headache, chills, fever, asthenia. Pallor, diarrhea, nausea, vomiting, arthralgia, myalgia, dizziness, hyperkinesia, abdominal or back pain, hypotension, hypertension, increased LDH, somnolence, vasodilation, pruritus, rash and sweating. In the treatment of ITP, the most common adverse reactions (≤2% of infusions) occurring in the clinical trial were headache, chills, and fever.
Please see full Prescribing Information for complete prescribing details.
To report SUSPECTED ADVERSE REACTIONS, contact Kamada at pharmacovigilance@kamada.com or 1-(866)-916-0077 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.